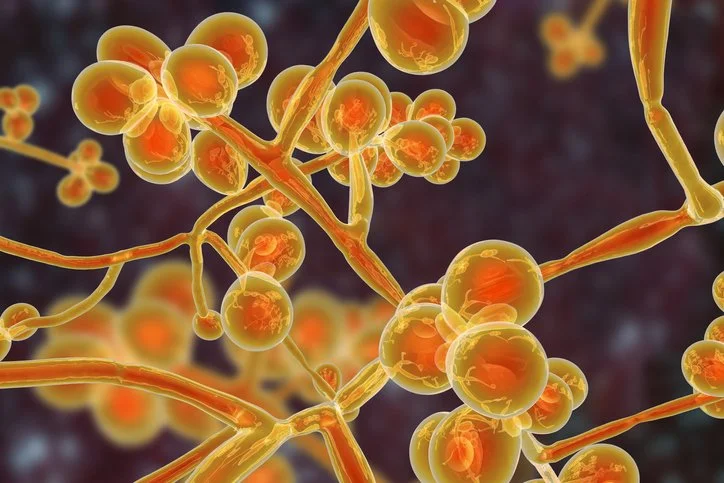
Candida auris, a multidrug resistant yeast, is the most recent infectious organism to spread in healthcare environments.
The Centers for Disease Control and Prevention estimates 80 percent of all infections are transmitted by hands.
Infection Prevention Services
1. We provide access to a multidisciplinary team including Physicians, Infection Preventionists and Nurses that are specialists in implementing Infection Prevention compliant programs.
2. Our team provides support to the facility’s designated infection preventionist providing tools and guidance necessary to implement a comprehensive Infection Prevention and Control Program including:
1. Quality Assurance and Performance Improvement (QAPI) exercises targeted at improving patient care and/or reducing costs
2. Quarterly Quality Assurance (QA) reporting
3. Our team provides an initial assessment of the facility’s current Infection Prevention and Control Program to identify areas for improvement and areas that are robust.
Provide a gap analysis report detailing deficiencies, areas for improvement and areas with sufficient rigor
Assist with facility-based and community-based risk assessment, utilizing an all-hazards approach including risk of MDROs and other communicable disease of interest (TB, Influenza, COVID, etc.) and utilizing facility surveillance activity and QAPI findings
Review standards, policies and procedures to ensure effectiveness and that they are in accordance with current standards of practice for preventing and controlling infections. This may include a review of changes in the facility population and characteristics that may identify components of the IPCP that must be changed accordingly.
4. Our team is able to guide the facility on serial Quality Assurance and Performance Improvement (QAPI) to help meet infection prevention related requirements for participation from Consolidated Medicare and Medicaid and local regulatory requirements, including but not limited to:
a. Hand Hygiene
b. Universal Precautions/Personal Protective Equipment
c. Surveillance (Process and Outcome) processes
d. System of Surveillance: Data Analysis, Documentation and Reporting
e. Processes for recognizing, containing and reporting Communicable Disease Outbreaks
f. Prevention and Control of Transmission of Infection
g. Medical Device Safety
h. Recording Infection Prevention and Control Program Incidents
i. Linen management
j. Urinary Catheter/UTI
k. Food management
l. Medication Administration
m. Environmental Services
n. Dental
o. Dialysis
p. Interfacility Communication for Admission and Discharge
5. Our team provides an Annual Review of the facility’s Infection Prevention and Control Program:
a. Review risk assessment, program plan, policies and procedures
b. Review Staff in services/education, including orientation and annual training
c. Review Outbreak management processes and policies
6. In conjunction with the facility’s Infection Preventionist, our team can help guide one QAPI exercise per year targeted at improving patient care and/or reducing costs
7. Our team will conduct an annual mock survey to engage front-line staff in real world scenarios, provide interpretation of regulations and standards, identify and eliminate deficiencies and inconsistencies and assist in the development of corrective action and improvement plans.
Test the facility’s ability to maintain a system of preventing, identifying, reporting, investigating and controlling infections and communicable diseases for all residents, staff, volunteers, visitors, and other individuals providing services
Provide a report within of mock survey results detailing deficiencies, areas for improvement and areas with sufficient rigor
8. Our team provides education to the facility on Infection Prevention that offers comprehensive guidance on how to properly apply “best practices” for Infection Prevention and Control including:
a. Best practices for education of patients and their families
b. Best practices for the nursing staff and the medical staff
c. Development of educational materials for use by the facility
d. Provide in-depth education sessions, as needed, to the facility’s staff
9. Our team will review data developed by the facility’s Infection Preventionist in preparation of one Quarterly QA meetings annually to provide an opinion on the comprehensiveness of data.
10. Our team can provide emergency consultation and outbreak management if requested.
11. Annual N95 Fit Testing and certification available.